Methotrexate (MTX) is used to fight a range of cancers in adults and children, including osteosarcoma, acute lymphoblastic leukemia, and several types of lymphoma.
Yet like any number of other life-saving therapies, it can also create complications in certain patients: when administered in high doses, those with some degree of renal failure can’t clear MTX fast enough, which in turn can cause severe toxicity. To complicate matters, MTX itself can cause kidney injury, leading to the delay of its own elimination from the body.
It’s an issue our antidote, Voraxaze (glucarpidase), can help solve. But because it happens so infrequently, it can be difficult for physicians, nurses and pharmacists to know when to administer this rescue medication. Delayed MTX elimination can be an oncologic emergency, but it is defined as occurring only when MTX concentrations are greater than 2 standard deviations of the mean MTX excretion curve specific for the MTX dose; this is not a measure that is readily ascertained from clinical experience. Clinicians also need to account for serum creatine levels (an indicator of kidney function), certain demographic information, and when the sample of MTX concentration was taken.
The bottom line? It’s an understandably difficult calculation to make for healthcare professionals, most of whom have likely not dealt with MTX toxicity before. How can we make MTX pharmacokinetics (PK) easier?
That’s where a new, web-based clinical decision support tool comes in.
Supported by an unrestricted grant from BTG Specialty Pharmaceuticals, a team from the Cincinnati Children’s Hospital Medical Center Department of Pharmacology – led by Dr. Laura Ramsey – created mtxpk.org to help monitor and predict delayed MTX clearance.
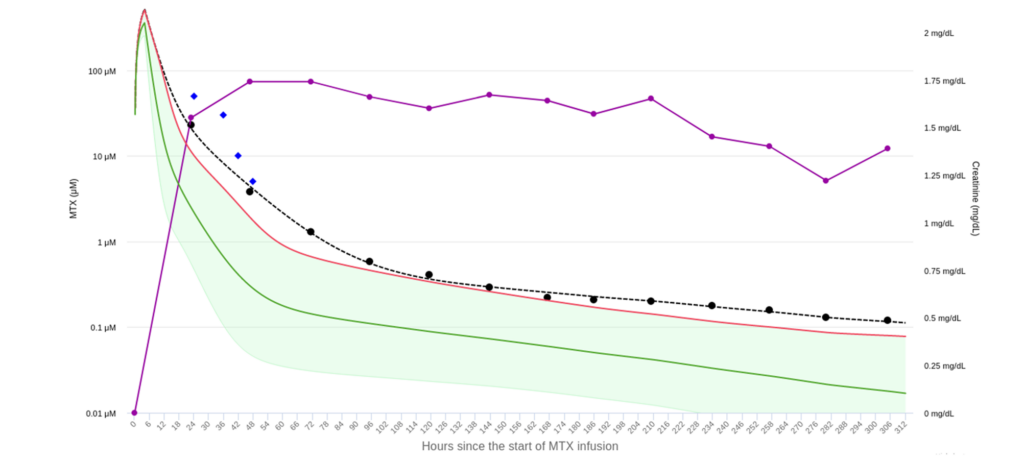
In a nutshell, here’s how it works: a clinician inputs the age, gender, height, and weight of the patient, the MTX dose and infusion duration, and at least one MTX plasma concentration measurement and one serum creatine measurement. From there, the tool will display the concentration vs. time curve (i.e. the PK curve) for that individual patient, overlaid upon the population-predicted curve for that dose. In doing so, it quickly provides model-informed guidance on the patient’s need for Voraxase, as well as an estimate of when the patient may reach the MTX concentration threshold necessary for hospital discharge.
Aside from its real-time, individualized guidance, the site is just another example of how novel data science is making its way into medicine. The model used to develop mtxpk.org, for instance, was based on 31,672 MTX plasma concentrations from 772 patients.
The web-based tool is free and open source. No patient data is stored upon use, however, it does allow clinicians to save data to their local device for future reference. A PDF of the patient’s PK curve can be downloaded, printed or saved.
Complications from high doses of MTX may be relatively rare – yet it’s this very rarity that can make it difficult to diagnose and know how to effectively treat. Fortunately this new, easy-to-use website has the potential to make all the difference.
The Role of High Dose Methotrexate
Learn more about methotrexate, an antifolate therapeutic agent that possesses potent anticancer activity against both solid tumors and leukemia.
INDICATION AND LIMITATIONS OF USE
- Voraxaze® is a carboxypeptidase indicated to reduce toxic plasma methotrexate concentration (greater than 1 micromole per liter) in adult and pediatric patients with delayed methotrexate clearance (plasma methotrexate concentrations greater than 2 standard deviations of the mean methotrexate excretion curve specific for the dose of methotrexate administered) due to impaired renal function
- Limitations of Use: Voraxaze® is not recommended for use in patients who exhibit the expected clearance and expected plasma methotrexate concentration. Reducing plasma methotrexate concentration in these patients may result in subtherapeutic exposure to methotrexate
IMPORTANT SAFETY INFORMATION
WARNINGS AND PRECAUTIONS
Serious Hypersensitivity Reactions
- Serious hypersensitivity reactions, including anaphylactic reactions, may occur. Serious hypersensitivity reactions occurred in less than 1% of patients
Monitoring Methotrexate Concentration/Interference with Assay
- Methotrexate concentrations within 48 hours following Voraxaze® administration can only be reliably measured by a chromatographic method due to interference from metabolites. Measurement of methotrexate concentrations within 48 hours of Voraxaze® administration using immunoassays results in an overestimation of the methotrexate concentration
ADVERSE REACTIONS
- In clinical trials, the most common related adverse events (occurring in >1% of patients) were paresthesia, flushing, nausea and/or vomiting, hypotension and headache
DRUG INTERACTIONS
- Voraxaze® can decrease leucovorin concentration, which may decrease the effect of leucovorin rescue unless leucovorin is dosed as recommended, and may also reduce the concentrations other folate analogs or folate analog metabolic inhibitors
Please see full Prescribing Information.


